New science for announcements: Musk informs the world about the condition of the first Neuralink patient
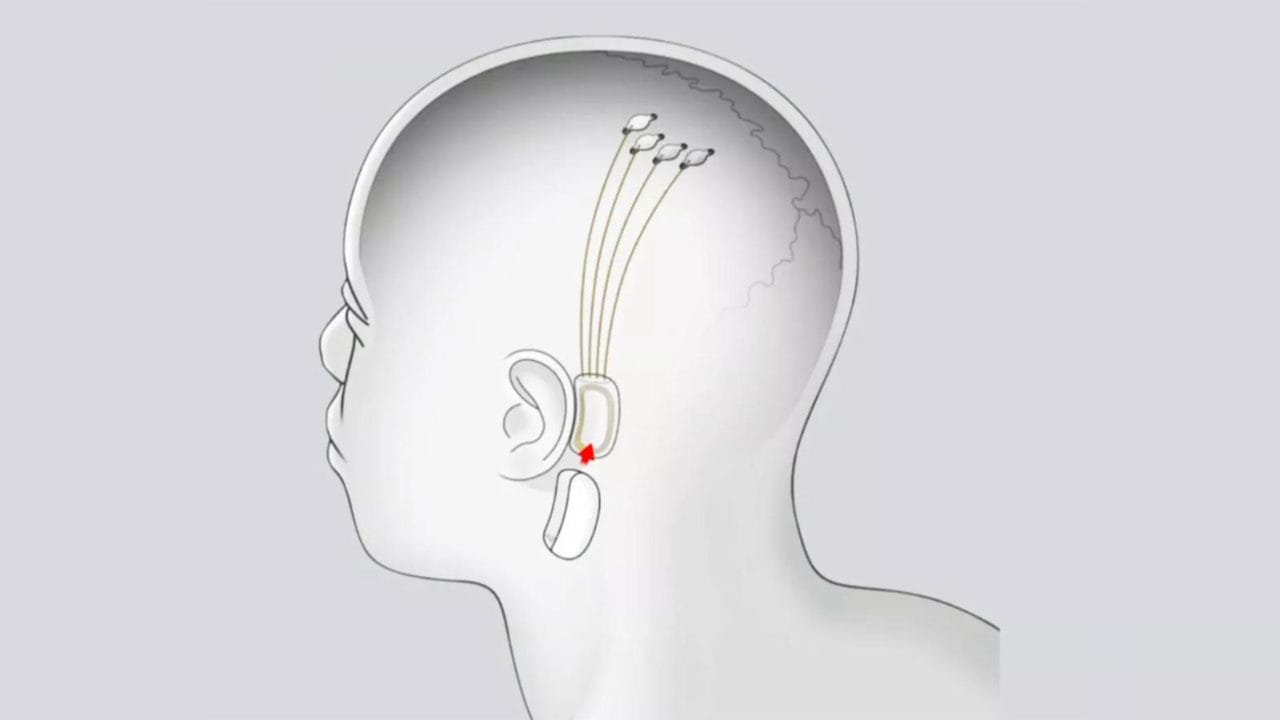
The announcement was made just a few weeks ago Neuralink of having implanted his first chip in the brain of a patient with motor problems, and yesterday Elon Musk announced that man is not alone is finebut is able to move the cursor a mouse with the thought (speaking of mice, do you know what dpi are and what they are for?).
The declaration happened Monday in a live event in one space on X, when asked for updates on the operation and the patient’s condition. “Progress is good and the patient appears to have made a full recovery, with no adverse effects that we are aware of. The patient is able to move a mouse on the screen just thinking“Musk said.
Obviously the news, despite Musk himself had anticipated that he did not want to use the event to provide information about it, it went around the world and caused a lot of outcry, as well as numerous criticisms.
Not that Neuralink is new to this type of advertising, but obviously the more you advance in this type of research, the greater it will be attention of the public and the scientific community.
Let’s remember that Musk’s company, strong of a financial support never seen, received the green light to experimentation human in May 2023, after passing a first refusal by the FDA (Food and Drug Administration) and a fine for violation of safety regulations on the transport of dangerous materials (as well as an ongoing investigation into the death of an unknown number of animals used as guinea pigs).
Neuralink never disclosed the identity of the patient, but last year he said he was looking for individuals with quadriplegia due to injuries of medulla cervical spinal or amyotrophic lateral sclerosiscommonly known as ALS or Lou Gehrig’s disease.
The study involves implanting a brain-computer interface into a part of the brain that transmits the intention to move, and at the end of January 2024 came the announcement of first plant.
Without articles or publications, just an announcement. Not illegal, but highly unusualin the field of science. So that the Hastings Centera non-profit institute that has contributed to the development of bioethics, called this situation “science for press release“, believing in a post that “an unprecedented experiment involving a vulnerable person” should include at least one formal reporting to the public.
Arthur Caplanchair of the Department of Medical Ethics at the University of Pennsylvania, e Jonathan Morenoprofessor of ethics, contested the project, saying in a statement that while the FDA does not require reporting, the doctors and scientists involved have the moral duty to provide transparency.
Even worse, they stated, “qhen the person who pays for a human experiment with a huge financial stake in the outcome [Elon Musk, NdR] is the only source of information, basic ethical standards have not been met“.
What’s worse is that there is no confirmation of the actual veracity of Musk’s claims, independent verification or otherwise, and, as Caplan and Moreno note, “A technical regulatory veil does not protect [gli scienziati] from the ethical obligations of transparency to avoid the risk of giving False hopes to countless thousands of people with severe neurological disabilities“.
At the moment Neuralink did not share further information about his page Xevidently the PubMed of the new science.